Sorry, I know this post is all about science, but I thought it could help some people who don’t know about this analytical technique (like me).
Inductively coupled plasma- Atomic emission spectrometry (ICP-AES)
(By Zoobia Nadeem)
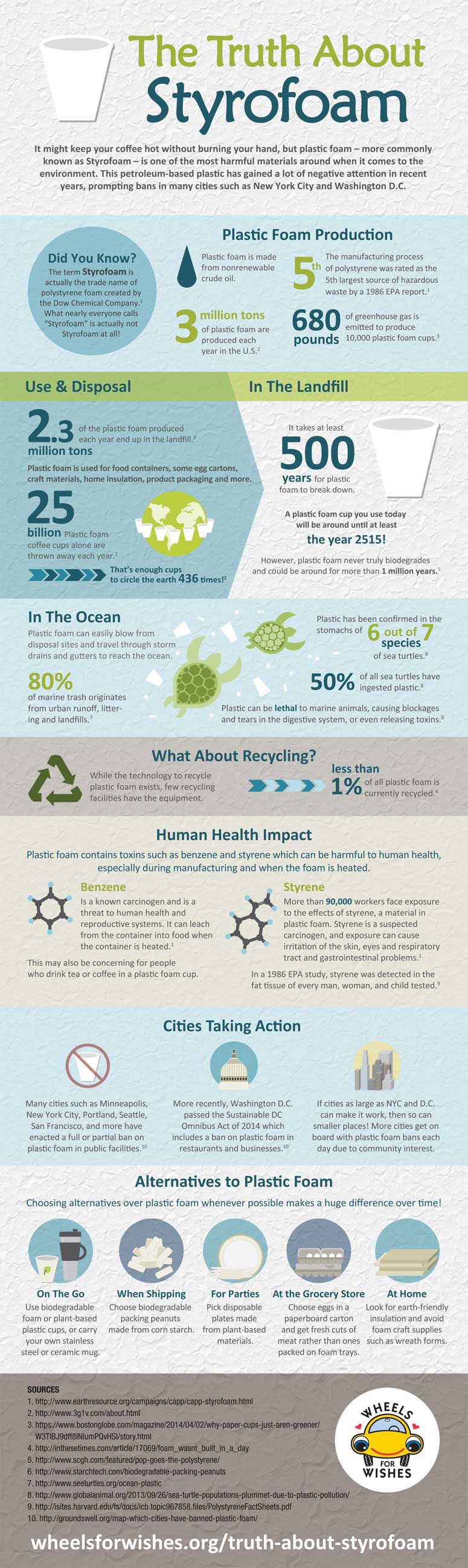
ICP-AES is a type of emission spectrometry designed for determination of the amount (concentration) of a test element in a sample. This characterization technique involves the atomization and excitation of the test element by inductively coupled plasma (ICP) and determination of amount (concentration) of test element from the intensity of atomic emission spectral line.
ICP-AES can detect more than 70 elements and offers several advantages over other techniques such as Atomic Absorption Spectroscopy. Some of these advantages include:
- Possibility of rapid and simultaneous multi-elemental analysis.
- Atomic emission source (ICP) is relatively free of chemical interference owing to its high temperature (6000 to 10,000 K).
- Low detection limits.
- Good accuracy and precisions.
- Elements that are difficult to be determined through AAS, such as Boron and Vanadium, can be measured (Charles & Ferdeen, 1997).
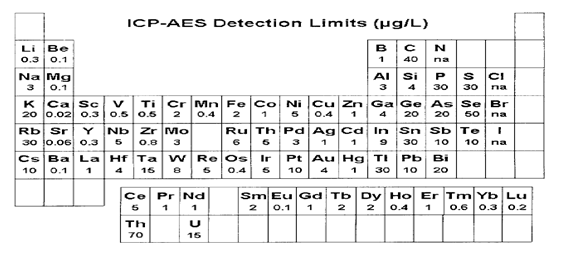
Figure 1: Elements of the periodic table which can be analyzed using ICP-AES (Charles & Ferdeen, 1997).
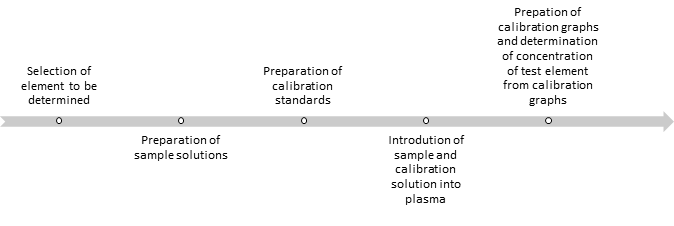
Figure 2: Steps in analysis of samples by ICP-AES (Agilent technologies, 1999).
1.1 Instrumentation
An ICP-AES instrument typically involves the following components:
1.1.1 Sample introduction system
Test sample is pumped into a nebulizer, where it is converted into a fine aerosol spray by a stream of argon (Ar) gas. This aerosol enters the plasma through inner tube of the plasma torch.
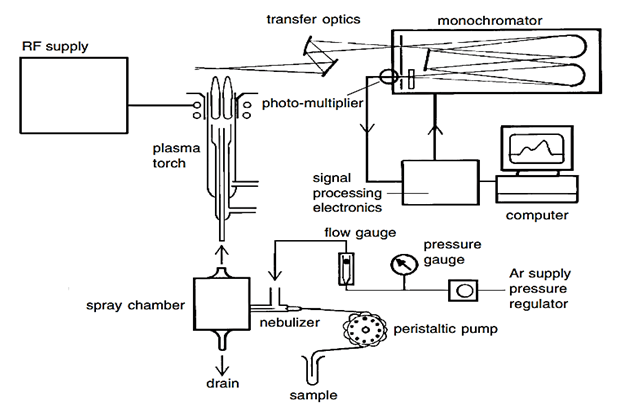
Figure 3: Components of ICP-AES (Agilent technologies, 1999).
1.1.2 The ICP discharge system
Following steps are involved in the generation of ICP:
- Ar gas is directed through a torch comprising three concentric tubes made of quartz or any other appropriate material. The upper portion of this torch is surrounded by a copper coil (load coil) which is connected to a radiofrequency generator.
- When RF power (700 to 1500 watts) is applied to the load coil, RF electric and magnetic fields are set up in the upper region of the torch.
- An electric charge is applied to the Ar gas circulating through the torch, which leads to the stripping of electrons from Ar atoms.
- The stripped electrons are caught up in and accelerated by magnetic field. The increase of energy of electrons by the use of coil in this manner is known as inductive coupling. The high energy electrons then collide with other Ar atoms and strip off more electrons.
- The process continuous in a chain reaction to generate an inductively coupled plasma consisting of electrons, Ar ions and Ar atoms. The functions of high temperature ICP discharge involve desolvation (removal of solvent to generate microscopic particles), vaporization (decomposition of particles into stream of individual molecules), atomization (disassociation into atoms), excitation and ionization of test sample (Charles & Ferdeen, 1997).
1.1.3 Optical Spectrometer
As mentioned above, in ICP-AES, light emitted by the excited atoms and ions in the plasma is measured to determine the composition of test sample. On the basis of mode of operation, ICP-AES can be divided into two types, which are simultaneous and sequential instruments. Simultaneous instruments detect several spectral lines simultaneously using a polychromater with a detector for each spectral lines. Sequential instruments are relatively more suited for laboratories as they allow the freedom of selection of a set of analytical wavelengths using some monochromater (Agilent technologies, 1999).
Both these types of ICP-AES instruments use focusing optics, a grating spectrometer and a detector. Focusing optics (lenses or mirrors) collect and focus the light emitted from the plasma onto the entrance slit of spectrometer. A grating spectrometer then generates a spectrum of the focused light so that the intensity of light can be measured at very precisely defined wavelengths. Specific wavelengths are then detected using photo-sensitive detectors such as photo-multiplier tube (PMT), charge-induction device (CID), or charge-coupled device (CCD) (Charles & Ferdeen, 1997).
1.1.4 Computers
Computers are used to perform several functions such as background correction, preparation of calibration graphs, calculation and analysis of results. These are also used to access databases and control the instrument (Agilent technologies, 1999).
1.2 Applications
Some of the applications of this technique have been listed in the following table (Agilent technologies, 1999):
| Field |
Applications |
| Agriculture, food and beverages |
Determination of micronutrient and toxic elements in agricultural
samples such as soil, plant tissue, grains, forages, animal feeds,
fertilizers, milk, etc. |
| Biology |
Determination of essential elements and non-essential elements in human urine, blood, soft and hard tissues, animal samples. |
| Geology |
Determination of minerals and radioactive elements in rocks, minerals, ores, concentrates, coal, fly ash, etc. |
| Water |
For the monitoring and analysis of surface water, freshwater, drinking water, etc. |
| Organics |
Determination of metals in cooking oils, antifreeze, pesticides, etc. |
2 References
Charles, B., & Fredeen, K. J. (1997). Concepts, instrumentation and techniques in inductively
coupled plasma optical emission spectrometry. Shelton, CT: Perkin Elmer Corporation.
Agilent technologies. (1999). Liberty II: Analytical methods book. Victoria, VIC: Varian
Australia Private Limited.


















 I was also going to mention the goals I have for 2018, but I think the readers might get bored.
I was also going to mention the goals I have for 2018, but I think the readers might get bored.